
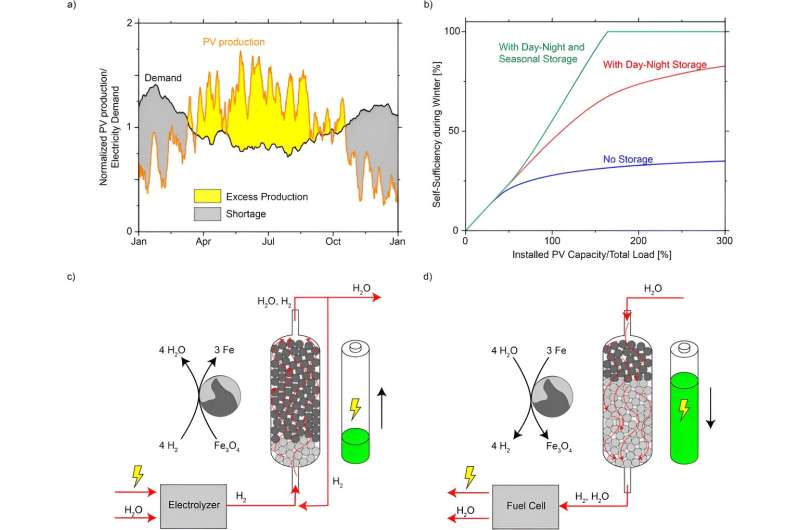
Photovoltaics are set to meet over 40% of Switzerland's electricity needs by 2050. But solar power isn't always available when it's needed: there's too much of it in summer and too little in winter, when the sun shines less often and heat pumps are running at full tilt. According to the Swiss federal government's Energy Strategy, Switzerland wants to close the winter electricity gap with a combination of imports, wind and hydropower as well as alpine solar plants and gas-fired power plants.
One way to minimize the need for imports and gas-fired power plants in winter is to produce hydrogen from cheap solar power in summer, which could then be converted into electricity in winter. However, hydrogen is highly flammable, extremely volatile and makes many materials brittle.
Storing the gas from summer until winter calls for special pressurized containers and cooling technology. These require a lot of energy, while the many safety precautions that must be followed make building such storage facilities very expensive. What's more, hydrogen tanks are never completely leak-proof, which harms the environment and adds to the costs.
Now researchers at ETH Zurich led by Wendelin Stark, Professor of Functional Materials at the Department of Chemistry and Applied Biosciences, have developed a new technology for the seasonal storage of hydrogen that is much safer and cheaper than existing solutions. The researchers are using a well-known technology and the fourth most abundant element on Earth: iron.
The findings are published in the journal Sustainable Energy & Fuels.
Chemical storage
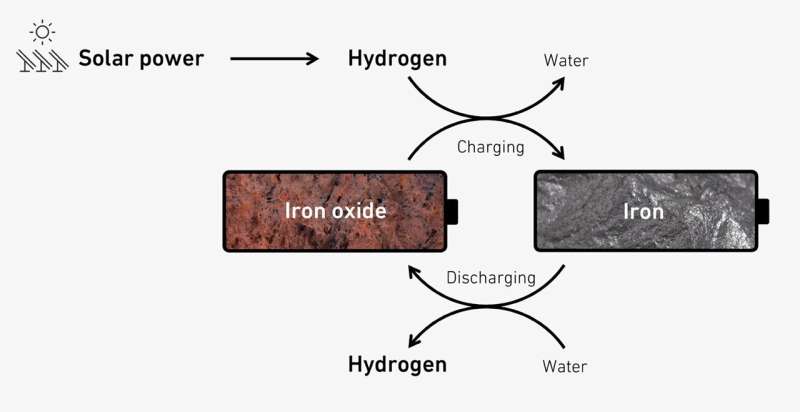
To store hydrogen better, Stark and his team are relying on the steam-iron process, which has been understood since the 19th century. If there is a surplus of solar power available in the summer months, it can be used to split water to produce hydrogen. This hydrogen is then fed into a stainless steel reactor filled with natural iron ore at 400 degrees Celsius. There, the hydrogen extracts the oxygen from the iron ore—which in chemical terms is simply iron oxide—resulting in elemental iron and water.
"This chemical process is similar to charging a battery. It means that the energy in the hydrogen can be stored as iron and water for long periods with almost no losses," Stark says.
When the energy is needed again in winter, the researchers reverse the process: they feed hot steam into the reactor to turn the iron and water back into iron oxide and hydrogen. The hydrogen can then be converted into electricity or heat in a gas turbine or fuel cell. To keep the energy required for the discharging process to a minimum, the steam is generated using waste heat from the discharging reaction.
Cheap iron ore meets expensive hydrogen
"The big advantage of this technology is that the raw material, iron ore, is easy to procure in large quantities. Plus it doesn't even need processing before we put it in the reactor," Stark says. Moreover, the researchers assume that large iron ore storage facilities could be built worldwide without substantially influencing the global market price of iron.
The reactor in which the reaction takes place doesn't have to fulfill any special safety requirements either. It consists of stainless steel walls just 6 millimeters thick. The reaction takes place at normal pressure and the storage capacity increases with each cycle.
Once filled with iron oxide, the reactor can be reused for any number of storage cycles without having to replace its contents. Another advantage of the technology is that the researchers can easily expand the storage capacity. It's simply a case of building bigger reactors and filling them with more iron ore. All these advantages make this storage technology an estimated ten times cheaper than existing methods.
However, there's also a downside to using hydrogen: its production and conversion are inefficient compared to other sources of energy, as up to 60% of its energy is lost in the process. This means that as a storage medium, hydrogen is most attractive when sufficient wind or solar power is available and other options are off the table. That is especially the case with industrial processes that can't be electrified.
Pilot plant on the Hönggerberg campus
The researchers have demonstrated the technical feasibility of their storage technology using a pilot plant on the Hönggerberg campus. This consists of three stainless steel reactors with a capacity of 1.4 cubic meters, each of which the researchers have filled with 2–3 metric tons of untreated iron ore available on the market.
"The pilot plant can store around 10 megawatt hours of hydrogen over long periods. Depending on how you convert the hydrogen into electricity, that'll give you somewhere between 4 and 6 megawatt hours of power," explains Samuel Heiniger, a doctoral student in Stark's research group. This corresponds to the electricity demand from three to five Swiss single-family homes in the winter months. At present, the system is still running on electricity from the grid and not on the solar power generated on the Hönggerberg campus.
This is soon set to change: the researchers want to expand the system such that by 2026, the ETH Hönggerberg campus can meet one-fifth of its winter electricity requirements using its own solar power from the summer. This would require reactors with a volume of 2,000 cubic meters, which could store around 4 gigawatt hours (GWh) of green hydrogen.
Once converted into electricity, the stored hydrogen would supply around 2 GWh of power. "This plant could replace a small reservoir in the Alps as a seasonal energy storage facility. To put that in perspective, it equates to around one-tenth of the capacity of the Nate de Drance pumped storage power plant," Stark says. In addition, the discharging process would generate 2 GWh of heat, which the researchers want to integrate into the campus's heating system.
Good scalability
But could this technology be harnessed to provide seasonal energy storage for Switzerland as a whole? The researchers have made some initial calculations: providing Switzerland with around 10 terawatt hours (TWh) of electricity from seasonal hydrogen storage systems every year in the future—which would admittedly be a lot—would require some 15–20 TWh of green hydrogen and roughly 10,000,000 cubic meters of iron ore.
"That's about 2% of what Australia, the largest producer of iron ore, mines every year," Stark says. By way of comparison, in its Energy Perspectives 2050+, the Swiss Federal Office of Energy anticipates total electricity consumption of around 84 TWh in 2050.
If reactors were built that could store around 1 GWh of electricity each, they would have a volume of roughly 1,000 cubic meters. This calls for around 100 square meters of building land. Switzerland would have to build some 10,000 of these storage systems to obtain 10 TWh of electricity in winter, which corresponds to an area of around 1 square meter per inhabitant.
More information: Samuel P. Heiniger et al, Safe seasonal energy and hydrogen storage in a 1 : 10 single-household-sized pilot reactor based on the steam-iron process, Sustainable Energy & Fuels (2023). DOI: 10.1039/D3SE01228J
Citation: Pilot plant demonstrates iron-based hydrogen storage feasibility (2024, August 31) retrieved 2 September 2024 from https://techxplore.com/news/2024-08-iron-based-hydrogen-storage-feasibility.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
