High energy density is a crucial direction for future battery development. Lithium-sulfur (Li-S) batteries, with their high theoretical energy density, have garnered significant attention. However, the slow solid-liquid-solid conversion of sulfur, especially the oxidation of lithium sulfide (Li2S) during charging, which requires overcoming large reaction barriers, leads to incomplete Li2S conversion and electrode passivation.
As a result, the energy density and cycle performance of the batteries still fall short of commercial requirements. Recently, introducing catalysis has become an effective strategy to enhance cathode kinetics and increase sulfur utilization. However, the limited contact and weak interaction between solid-phase catalysts and solid Li2S severely confine the efficient reversible conversion of sulfur, especially under high-sulfur loading and lean electrolyte, and thus greatly restricting the energy density and cycle stability of Li-S batteries.
This study is led by Prof. Lv Wei (Tsinghua Shenzhen International Graduate School), and Prof. Yang Quan-Hong (Tianjin University, Joint School of National University of Singapore and Tianjin University). The collaboration group develops an electrochemical molecular imprinting technology suitable for Li-S batteries through the irreversible delithiation properties of metal sulfides (MS).
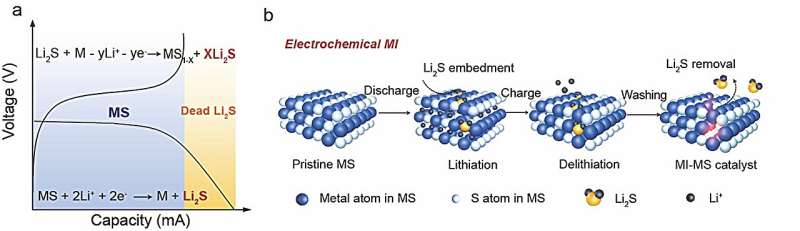
Specifically, Li2S imprinting defects were constructed in MS by pre-embedding Li2S with lithiation/delithiation process, and removing Li2S by alcohol washing. The structural characterization showed that the sulfur vacancy formed in the catalyst due to the removal of Li2S. This special defect allows the catalyst to selectively bind to the target product Li2S.
The paper is published in the journal National Science Review.
The researchers also demonstrated the universality of the method by testing different MSs, and the catalyst performance is positively correlated with the sulfur vacancy content, indicating that the defect customized for Li2S in MSs can significantly promote the reaction. After materials screening, the targeted adsorption effect of MI-Ni3S2 on Li2S was demonstrated by QCM, and the high catalytic conversion effect of Li2S oxidation was proved by Li2S activation potential experiment.
Further, the mechanism was elucidated by DFT: such tailor-made defects enable the catalyst to bind exclusively to Li atoms in Li2S reactant and elongate the Li-S bond, thus decreasing the reaction energy barrier during charging and finally expediting the conversion of Li2S to sulfur.
In terms of battery performance, under practical conditions, the assembled Ah-scale Li-S pouch cell cycled stably over 100 cycles, achieving an energy density exceeding 300 Wh/kg based on the total mass. Furthermore, under extremely low electrolyte (E/S=1.8 μL/mgS), the team successfully developed batteries with an energy density of 502 Wh/kg using this catalyst, surpassing the performance of most currently reported works.
To conclude, the proposed synthetic approach offers an ideal solution for the tricky Li2S dissociation problem that is critical for the final industrialization of Li-S batteries. More promisingly, this work provides an effective way and, more importantly, a rationale to synthesize practical catalysts with a well-managed solid-solid interfacing not limited to high-energy sulfur-based batteries.
More information: Yufei Zhao et al, Engineering catalytic defects via molecular imprinting for high energy Li-S pouch cells, National Science Review (2024). DOI: 10.1093/nsr/nwae190
Citation: Electrochemically molecular-imprinted catalysts enable high-energy-density Li-S batteries (2024, June 28) retrieved 28 June 2024 from https://techxplore.com/news/2024-06-electrochemically-molecular-imprinted-catalysts-enable.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
