Hydrogen fuel cells, devices that can convert the chemical energy stored in hydrogen into electrical energy via an electrochemical reaction, are promising solutions for electrifying large vehicles. Fuel cells based on low-temperature (under 100 °C) proton exchange membranes have been found to be particularly advantageous for transportation, as they produce limited noise, have high power densities and can power vehicles for long distances with a single charge of hydrogen.
Despite their promise, these low-temperature fuel cells only operate well with highly pure hydrogen, as well as sophisticated heat and water management systems, which could limit their real-world use. Raising the operating temperature of fuel cells to 120–150 °C could help reduce these requirements, making them more tolerant to hydrogen impurities while also simplifying their internal cooling and water management.
Researchers at the Korea Institute of Science and Technology recently designed a new polymer electrolyte membrane (PEM) for fuel cells that can operate at temperatures above 200 °C and up to 250 °C. Their proposed PEM, presented in a paper published in Nature Energy, is based on a unique type of proton carrier, which can be deployed as a self-assembled network to promote proton conduction.
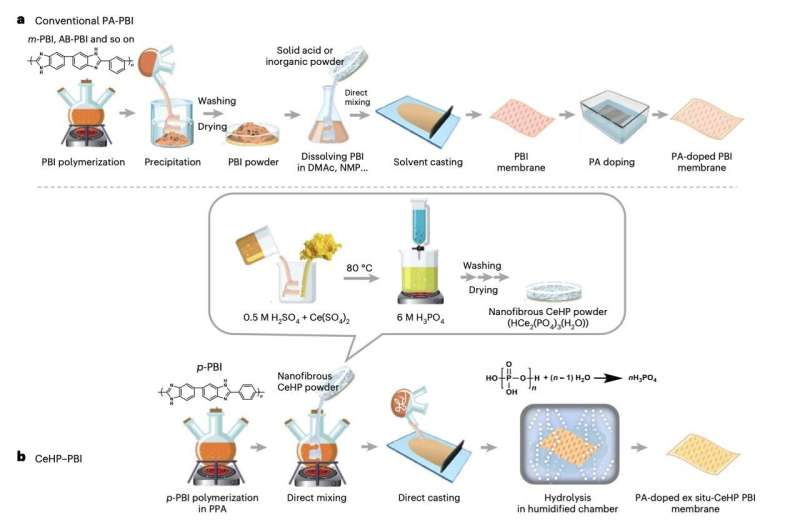
"Operating PEM fuel cells at high temperatures can simplify water management and allow integration with high-purity fuel processing units," Seungju Lee, Jong Geun Seong and their colleagues wrote in their paper. "However, existing polybenzimidazole (PBI)-based PEM fuel cells face challenges due to the instability of proton transport above 160 °C. We report a PEM composed of para-PBI (p-PBI) and cerium hydrogen phosphate (CeHP) that can be used in a fuel cell at up to 250 °C."
The researchers showed that at temperatures above 200 °C, the primary proton conduction of their PEM changed in a way that allowed fuel cells to operate at temperatures of 250 °C. In initial tests, a fuel cell based on this membrane was found to attain a remarkable electrochemical performance, and a boosted tolerance to CO and greater duration of operation compared to other existing fuel cells operating at these high temperatures.
"During fabrication, echinoid-shaped CeHP particles form a well-dispersed and interconnected self-assembled network within the PBI matrix (SAN–CeHP–PBI), allowing them to outperform p-PBI and conventional CeHP–PBI PEMs in terms of proton transport above 200 °C," Lee, Seong and their colleagues wrote.
"We report a SAN–CeHP–PBI-based fuel cell that reaches a maximum power density of 2.35 W cm−2 (at 250 °C in dry H2/O2) with negligible degradation over 500 h during thermal cycling (at 160–240 °C, H2/air). SAN–CeHP–PBI also demonstrates excellent CO tolerance, showing promise for integration with liquid hydrogen carrier systems."
This recent study by Lee, Song and his colleagues could soon open new possibilities for the development of better performing fuel cells for transportation-related applications. While the team's new PEM has already achieved promising results, its commercialization will only be possible after a series of technical issues are overcome. Most notably, scientists and engineers will first have to identify stable catalysts and binders that can tolerate prolonged exposure to temperatures above 250 °C.
More information: Seungju Lee et al, Self-assembled network polymer electrolyte membranes for application in fuel cells at 250 °C, Nature Energy (2024). DOI: 10.1038/s41560-024-01536-4
© 2024 Science X Network
Citation: New polymer electrolyte membranes for fuel cells can operate at up to 250 °C (2024, June 20) retrieved 20 June 2024 from https://techxplore.com/news/2024-06-polymer-electrolyte-membranes-fuel-cells.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
