Organic solar cells (OSCs) are a key part of the global effort to end our reliance on fossil fuels. Over the past few decades, new advances in technology have led to steady improvements in their efficiency and affordability.
One particularly promising approach to boosting their performance is to use specialized polymers named "polyelectrolytes." So far, however, these materials have proven difficult to produce with the levels of purity required for use in OSCs.
Through new research published in Organic Electronics, Joo Hyun Kim and colleagues at Pukyong National University, Busan, South Korea, introduce a simple new approach for synthesizing high-purity polyelectrolytes, and applying them to OSCs.
"Such an innovation holds the promise of revolutionizing the field by offering a more accessible and efficient means of enhancing the performance of organic solar cells," Kim says.
Polyelectrolytes are produced through the assembly of positively and negatively charged ions dissolved in a solution. Previously, researchers showed how OSC efficiency can be improved by using the polymers as the "cathode interlayer": the thin layer of material placed between the cathode—where electrons flow out of the cell—and its active layer where incoming sunlight is converted into electricity.
This boost in efficiency occurs due to the unique electronic structure of polyelectrolytes, which enhances the collection of electrons generated in the active layer, while lowering the resistance of the flow of electrons from the active layer to the cathode.
"In the approach to polyelectrolyte production that is currently prevalent, the removal of excess starting materials remains a laborious and time-intensive task," Kim explains. "Consequently, the simpler purification method that we propose heralds a promising avenue for improving the efficiency of organic solar cells."
In their study, Kim's team employed a novel chemical process to assemble polyelectrolytes without any need for excess starting materials.
"The polyelectrolytes we used incorporate an ionic group into the polymer side chain, rendering them soluble in alcohol," Kim describes. "We introduced an ion exchange technique for modifying these polymers, aiming to utilize them as cathode interlayers in OSCs."
In this simple process, the negative charges associated with the dissolved polyelectrolyte were exchanged with different charges in the surrounding alcohol solution. This enabled the researchers to adjust the interactions between the ions of the dissolved polymer, which would determine the properties of the polyelectrolyte once these molecular building blocks joined together.
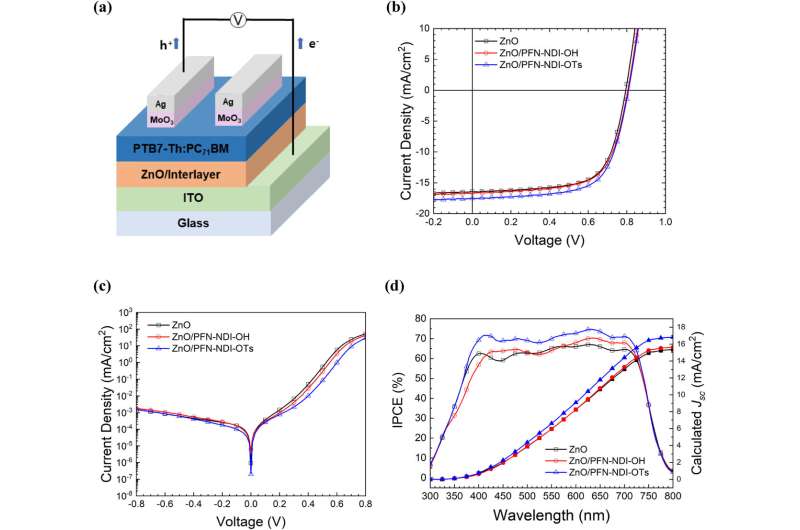
Altogether, this approach allowed Kim's team to tailor a strong performance in the OSC's cathode interlayer without any need for time-consuming purification. In their experiments, it enabled them to boost the efficiency of conversion from sunlight to electricity by over 9%.
"The results suggest that these polyelectrolytes show great potential for serving as a cathode interlayer in organic solar cells," Kim says. "They also offer the added benefit of enhancing interfacial properties through a straightforward anion exchange process, eliminating the need for complicated purification steps."
The team now hopes that their approach could be applied more widely, and so could accelerate the global rollout of renewable solar energy and reduce still further our reliance on fossil fuels.
More information: Rahmatia Fitri Binti Nasrun et al, A simple approach for improving the photovoltaic efficiency of organic solar cells through polymer modification via anion exchange as the interlayer, Organic Electronics (2024). DOI: 10.1016/j.orgel.2024.106995
Citation: New chemical synthesis technique could improve organic solar cell efficiency (2024, June 17) retrieved 17 June 2024 from https://techxplore.com/news/2024-06-chemical-synthesis-technique-solar-cell.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
