As a grid-scale energy storage system, flow batteries have gained increasing attention as a means to address the challenges associated with fluctuations and intermittency in renewable energy sources.
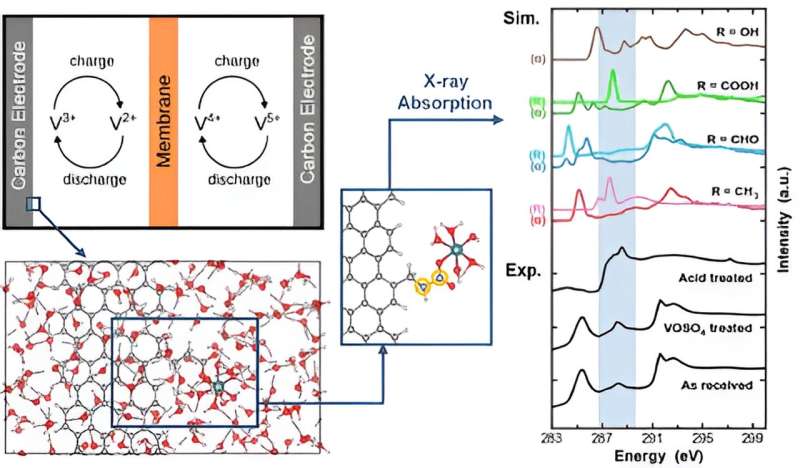
Vanadium redox flow batteries (VRFBs) have emerged as promising solutions for stationary grid energy storage due to their high efficiency, scalability, safety, near-room-temperature operation conditions, and the ability to size power and energy capacities independently. The redox reactions in a redox flow battery occur at the surfaces of the electrodes in contact with the electrolyte. Any modifications to the electrode surface can affect the electrochemical activity and affect the overall battery performance.
In an effort to extend the lifespans of VRFBs, Lawrence Livermore National Laboratory (LLNL) scientists and collaborators from the Pacific Northwest National Laboratory (PNNL) have explored the surface functionality of carbon electrodes and their propensity for degradation during electrochemical cycles.
The team conducted a coupled experimental–theoretical approach based on carbon K edge X-ray absorption spectroscopy (XAS) to characterize carbon electrodes prepared under different conditions and identify relevant functional groups that contribute to unique spectroscopic features.
"Our research identified the active species present on the carbon electrodes, which play a crucial role in determining their electrochemical performance," said LLNL researcher Wenyu Sun, the first author of a paper appearing in the journal ACS Applied Materials and Interfaces.
Sun said that XAS was ideal for the experiments because it is a particularly powerful method for investigating carbon electrode composition as it provides element-specific local electronic structure (and, therefore, bonding environment) as well as the capability for depth profiling, which enables comparison of the surface and bulk electrode composition.
Led by Sabrina Wan, a computational material scientist from LLNL's Quantum Simulation Group and a corresponding author of the paper, the team conducted density functional theory (DFT) calculations to obtain XAS signatures of relevant carbon atoms present in the functionalized carbon electrode, both before and after interaction with the vanadium redox complexes in different charge states.
The thermodynamically most favorable interaction and the corresponding chemical environment were identified by sampling a vast variety of atomic configurations from DFT and ab initio molecular dynamics. A library of XAS spectra was obtained for the carbon atoms residing in distinct chemical environments, and the team demonstrated that these simulated spectra are very powerful for deconvolving ex-situ experimental spectra collected for the carbon electrodes prepared under different conditions.
"This work will contribute to a deeper understanding of the electrochemical reactions occurring at the electrode-electrolyte interface, ultimately aiding in the design and optimization of high-efficiency VRFBs," said LLNL co-principal investigator Jon Lee, a corresponding author of this paper.
More information: Wenyu Sun et al, Coupled Experimental–Theoretical Characterization of a Carbon Electrode in Vanadium Redox Flow Batteries using X-ray Absorption Spectroscopy, ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.3c17049
Citation: Going with the flow: Research dives into electrodes on energy storage batteries (2024, April 24) retrieved 24 April 2024 from https://techxplore.com/news/2024-04-electrodes-energy-storage-batteries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
