Lithium-ion batteries (LIBs), mainly used as the power of computer, communication and consumer electronic products, require higher energy density, longer cycling life, faster-charging capability, and a broader operating temperature range to meet the growing consumer demands.
LiCoO2 (LCO) is the primary cathode material for LIBs. Currently, the advanced electrolytes for LCO cannot meet the high energy density and fast-charging performance of LIBs.
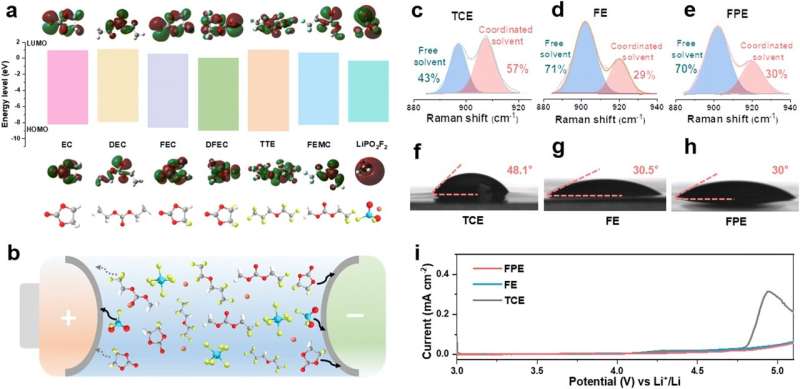
Recently, a research group led by Prof. Wu Zhongshuai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a novel universal additive-containing "cocktail electrolyte" based on the synergistic cooperation of multi-component additives. This electrolyte enabled commercial LCO with high voltage (4.6 V) and ultra-fast charging (5 C) in a wide temperature range (-20 to 45o C). It also exhibited high applicability to high-Ni and Co-free cathodes.
The study was published in Energy & Environmental Science.
In principle, increasing the charging cutoff voltage can improve the energy density of the batteries. However, it can lead to the continuous oxidative decomposition of electrolytes, excessive growth of non-uniform cathode-electrolyte interphase (CEI), and sluggish interfacial kinetics, which hinders LCO from achieving high voltage and fast charging.
To solve the above problems, the researchers proposed a novel "cocktail electrolyte" (FPE), which could improve the ultra-stable fast-charging cycle stability of commercial LCO at 4.6 V.
They revealed that the cooperation between multiple components in FPE led to the robust and kinetically fat electrode/electrolyte interphases on both cathode and anode. These interfaces, enriched with LiF and Li3PO4, displayed strong mechanical stability and enhanced ionic conductivity.
As a result, they prevented cathode surface degradation, suppressed unwanted interfacial reactions, accelerated reaction kinetics, and mitigated the formation of lithium dendrites even under extremely high current densities. Therefore, they achieved a high-performance 4.6 V Li-ion battery.
The results showed that the capacity retention in FPE was as high as 73.2%, even at 5 C over 1,000 cycles. In practical pouch-type cells, this electrolyte enabled graphite||LCO battery to maintain up to 72.1% capacity retention after 2,000 cycles and long-term cyclability over 3,800 cycles.
In addition, the researchers showed the general application of FPE in high-voltage Ni-rich and Co-free cathodes.
"This work provides a practical strategy for high-energy-density and fast-charging batteries," said Prof. Wu.
More information: Anping Zhang et al, Regulating electrode/electrolyte interfacial chemistry enables 4.6 V ultra-stable fast charging of commercial LiCoO2, Energy & Environmental Science (2024). DOI: 10.1039/D4EE00676C
Citation: Universal 'cocktail electrolyte' developed for 4.6 V ultra-stable fast charging of commercial lithium-ion batteries (2024, April 18) retrieved 18 April 2024 from https://techxplore.com/news/2024-04-universal-cocktail-electrolyte-ultra-stable.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
