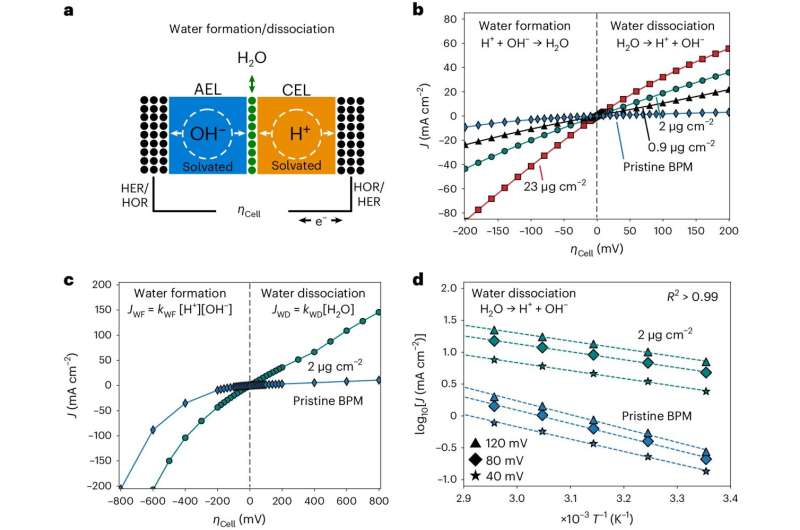
Bipolar membranes are a class of ion-conductive polymers comprised of two oppositely charged layers, known as the cation-exchange and anion-exchange layer. These membranes are central to the functioning of various technologies, including electrolyzers and hydrogen fuel cells.
While many companies and start-ups are using bipolar membranes to develop new energy technologies, their underlying working principles and ion solvation kinetics have not yet been fully elucidated. Better understanding these principles could inform the future fabrication of these materials and facilitate their successful integration in various devices.
Researchers at Fritz-Haber Institute of the Max Planck Society recently carried out a study to examine the water dissociation and ion solvaton kinetics at the interface between the two layers in bipolar membranes. Their paper, published in Nature Energy, gathered valuable new insight that could guide the future design of these membranes and of promising electrocatalysts for fuel cells.
"We wanted to understand the fundamental working principles of bipolar membranes and how they connect to electrochemistry more broadly," Sebastian Oener, corresponding author of the study, told Tech Xplore. "Bipolar membranes exist for over 65 years, but previous explanations about their working principles were rather unsatisfactory. We wanted to change that, connecting two fields that were previously thought to be separate."
To conduct their study, Carlos Gomez Rodellar, first author and Ph.D. student in the Interface Science Department, had to tackle various research challenges in an experimental setting. Firstly, he had to setup and benchmark a system that would allow him to study the kinetics of bipolar membranes without undesirable interferences from the cross-over of electrolyte ions.
"This system had to apply continuously physical pressure on the membrane electrode assembly with the metal oxide catalysts inside the bipolar junction," Oener explained. "Finally, Carlos had to design the whole system in such a way that we could controllably change the temperature of the cell and the humidified gases to do Arrhenius analysis and extract the bias dependent activation entropy and enthalpy. All of this can be provided with a modified fuel cell test station."
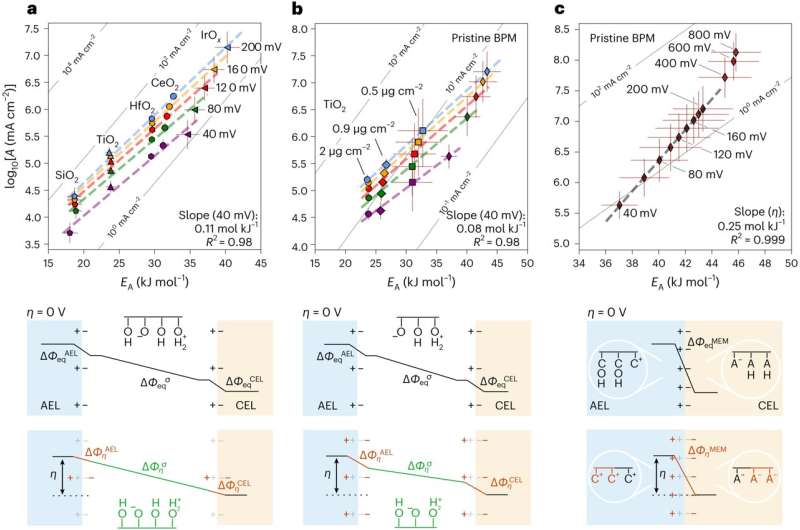
The measurements collected by the researchers paint a comprehensive picture of the fundamental principles underpinning the functioning of bipolar membranes. Specifically, they unveiled bias-dependent relationships between the activation entropy and enthalpy inside the bipolar junction, which appears to be related to a bias dependent dispersion of interfacial capacitance.
The team also observed that solvation kinetics in bipolar membranes exhibit characteristics that are unrelated to the chemical composition of the catalysts employed, but are likely originating from entropic changes in the interfacial electrolyte. Collectively, these insights could help to develop better performing bipolar membranes for electrodialysis, CO2 electrolyzers and H2 fuel cells.
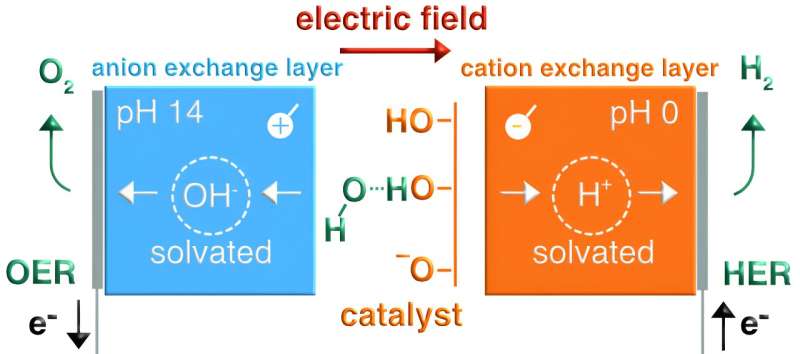
"There are many different applications of bipolar membranes that are being explored around the world, including at promising start-ups," Oener said. "There is really a lot of potential. Beyond bipolar membranes, we were also able to show that the same physics are at play when water is dissociated and hydroxide ions solvated at electrocatalyst interfaces."
The results gathered by the research group at the Interface Science Department of the Fritz Haber Institute demonstrate the importance of entropic changes on the solvent side at liquid-solid interfaces. Their work could thus also guide the design of new promising electrocatalysts to initiate specific chemical reactions, such as those required to generate green hydrogen from alkaline electrolytes.
"Regarding bipolar membranes, there are still open fundamental questions that we want to address," Oener added.
"For example, the water formation reaction is important when bipolar membranes are run in forward direction. We also explore several applications in collaboration with others. These include different types of fuel cells and electrodialysis type systems. When it comes to ion solvation in electrocatalysis, we have multiple projects that are either already in review or are getting close to be submitted. Stay tuned for more."
More information: Carlos G. Rodellar et al, Ion solvation kinetics in bipolar membranes and at electrolyte–metal interfaces, Nature Energy (2024). DOI: 10.1038/s41560-024-01484-z.
© 2024 Science X Network
Citation: New insight about the working principles of bipolar membranes could guide future fuel cell design (2024, April 16) retrieved 16 April 2024 from https://techxplore.com/news/2024-04-insight-principles-bipolar-membranes-future.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
