Lithium-ion batteries stand out as one of the most prevalent rechargeable battery technologies in the present era. Within these batteries, lithium-cobalt oxides (LiCoO2) are widely used as the materials for positive electrodes or cathodes (the conductors through which electric current either enters or exits a substance). The cathode plays a pivotal role in lithium-ion batteries and influences their capacity, performance over many charge-discharge cycles, and ability to manage heat.
One major issue leading to the deterioration of these batteries is the creation of hydrogen through the splitting of water. Therefore, gaining insights into how hydrogen builds up and is removed in LiCoO2 can greatly enhance the efficiency and functioning of solid-state lithium-ion batteries. Furthermore, this knowledge can lead to new ways to recycle used lithium-ion batteries to utilize them for hydrogen storage and production through the process of water splitting at room temperature.
Now, in a recent study published in the International Journal of Hydrogen Energy and led by Professor Bun Tsuchiya from the Department of General Education of the Faculty of Science and Technology at Meijo University, a team of researchers has conducted a thorough investigation into the hydrogen uptake and loss in LiCoO2 cathode materials immersed in water at room temperature.
According to Prof. Tsuchiya, "My motivation is to achieve the production of hydrogen (H2) through water (H2O) splitting at room temperature using certain oxide ceramic materials. Usually, H is dissociated from H2O at around 2000 K. However, this is too much energy for effective H2 fuel production and for solving current environmental problems, such as long-term carbon dioxide emissions."
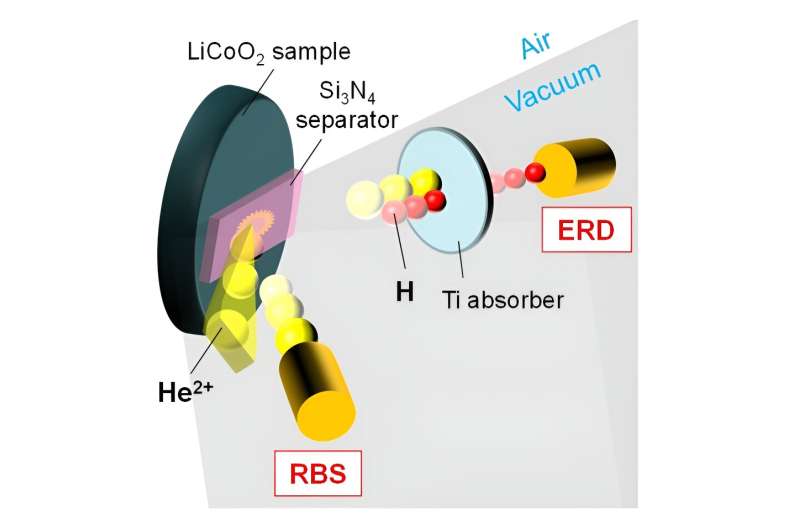
The study aimed to explore how LiCoO2 materials store and release hydrogen and identify the most stable locations within the LiCoO2 structure for trapping hydrogen. This was done using various analytical techniques, including weight gain and elastic recoil detection methods. The team revealed that the concentration of hydrogen increased after immersing the material in water for two minutes at specific temperatures.
Additionally, gas chromatography was used to analyze the release of hydrogen gas and determine the temperature at which dissociation occurred, which was found to be below 523 K. The study also involved density functional theory calculations, which indicated that hydrogen atoms separated from water tended to prefer lithium sites over other locations in the crystal structure of LiCoO2.
Overall, the results suggest that LiCoO2 has a significant role in storing hydrogen at room temperature through the process of water splitting to produce hydrogen gas. "If it becomes possible to make H2 from the inexhaustible H2O on earth with low energy input, I think that we can potentially establish a hydrogen-based society in the future," says Prof. Tsuchiya.
In summary, the researchers have investigated the storage and release of hydrogen in LiCoO2 cathode materials for lithium-ion batteries. By providing insights into a process that leads to degradation in this widely used technology, this study paves the way for the development of more efficient batteries as well as the low-energy production of hydrogen through water splitting, an environmentally friendly energy-storage technology.
More information: K. Kataoka et al, Hydrogen absorption and desorption characteristics of H2O-uptake LiCoO2 materials at room temperature, International Journal of Hydrogen Energy (2023). DOI: 10.1016/j.ijhydene.2023.10.039
Citation: Powering the future: Unlocking the role of hydrogen in lithium-ion batteries (2024, January 26) retrieved 26 January 2024 from https://techxplore.com/news/2024-01-powering-future-role-hydrogen-lithium.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
