As the use of electric and hybrid vehicles increases in many countries worldwide, the development of safe and better-performing battery technologies becomes increasingly crucial. Most notably, engineers have been trying to increase the safety and energy capacity of batteries while also ensuring their scalability and slowing down their degradation over time.
The battery technologies that could support the demands of the electronics industry include rechargeable multivalent metal batteries (i.e., batteries employing multivalent ions) based on anode materials with low-reduction potentials, such as magnesium (Mg) and calcium (Ca). These batteries could exhibit high energy densities if developed using the right combination of anodes, cathodes, and electrolytes.
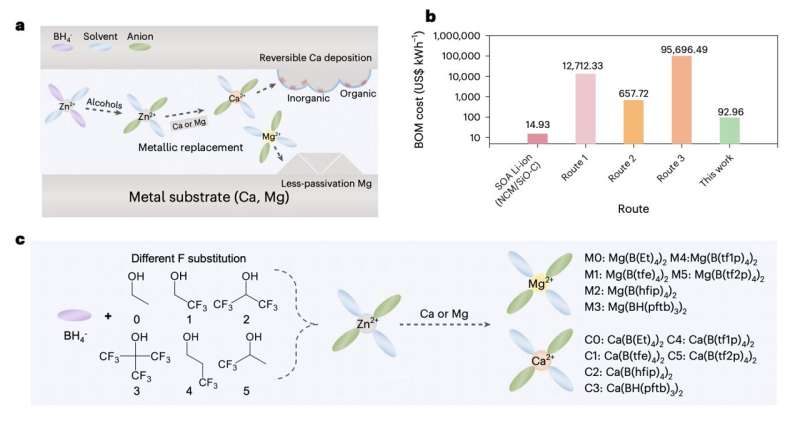
In recent years, studies have identified various cost-effective anode materials for these batteries. Many of the proposed electrolytes, on the other hand, are either difficult to source or rely on sophisticated synthesis processes, which makes them difficult to fabricate on a large scale.
Researchers at Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology recently introduced a new, universal method to realize highly performing and scalable electrolytes for multivalent metal batteries. Their proposed strategy, outlined in a paper in Nature Energy, could help to devise reversible and more affordable electrolyte systems, which could prove valuable for next-generation battery technologies.
"High-performance, cost-efficient electrolyte systems are sought after for high-energy-density multivalent metal batteries," Siyuang Li, Jiahui Zhang, and their colleagues wrote in their paper.
"However, the expensive precursor and complex synthesis process hinders exploration of cathode electrode/electrolyte interfaces and solvation structures. We developed a universal cation replacement method to prepare low-cost, high-reversibility magnesium and calcium electrolytes derived from a zinc organoborate solvation structure."
The method introduced by this research team spans various steps. Firstly, the researchers prompted a chemical reaction between an affordable and easily attainable Zn(BH4)2 precursor with different fluoroalcohols, producing target anions with various branched chains.
Subsequently, these anion solvates reacted with low-cost metal foils with a higher metal activity to produce target solvation structures. To suppress the continuous decomposition of solvents and maintain stable battery cycling, the researchers proposed the formation of a passivation layer based on two types of Ca solvates.
"By rationally adjusting the precursor chain length and F-substitution degree, we can fine-tune anion participation in the primary solvation shell," the researchers explained in their paper. "A completely dissociated Mg organoborate electrolyte enables high current endurance and enhanced electrochemical kinetics, whereas the Ca organoborate electrolyte with strong coordination/B–H inclusion offers a stable solid–electrolyte interphase with high coulombic efficiency."
The researchers have so far used their method to create a 53.4 Wh kg−1 high-loading battery prototype based on Mg/S, which contained a 30 μm Mg anode, a low electrolyte/ sulfur ratio (E/S = 5.58 μl mg−1) and a modified separator/interlayer. In initial tests, the battery prototype achieved promising results, highlighting the promise of this approach to create favorable and low-cost electrolytes for multivalent metal batteries.
In the future, the method introduced in this paper could pave the way toward the creation of various reversible electrolyte systems that rely on more affordable materials and simpler processing strategies. These electrolytes could be used to create scalable and safe multivalent metal batteries with higher energy densities.
More information: Siyuan Li et al, Cation replacement method enables high-performance electrolytes for multivalent metal batteries, Nature Energy (2024). DOI: 10.1038/s41560-023-01439-w
© 2024 Science X Network
Citation: A cation replacement method to realize highly preforming electrolytes for multivalent metal batteries (2024, January 21) retrieved 21 January 2024 from https://techxplore.com/news/2024-01-cation-method-highly-preforming-electrolytes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
