
Scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and Columbia University have developed a way to convert carbon dioxide (CO2), a potent greenhouse gas, into carbon nanofibers, materials with a wide range of unique properties and many potential long-term uses. Their strategy uses tandem electrochemical and thermochemical reactions run at relatively low temperatures and ambient pressure.
As the scientists describe in the journal Nature Catalysis, this approach could successfully lock carbon away in a useful solid form to offset or even achieve negative carbon emissions.
"You can put the carbon nanofibers into cement to strengthen the cement," said Jingguang Chen, a professor of chemical engineering at Columbia with a joint appointment at Brookhaven Lab who led the research. "That would lock the carbon away in concrete for at least 50 years, potentially longer. By then, the world should be shifted to primarily renewable energy sources that don't emit carbon."
As a bonus, the process also produces hydrogen gas (H2), a promising alternative fuel that, when used, creates zero emissions.
Capturing or converting carbon
The idea of capturing CO2 or converting it to other materials to combat climate change is not new. But simply storing CO2 gas can lead to leaks. And many CO2 conversions produce carbon-based chemicals or fuels that are used right away, which releases CO2 right back into the atmosphere.
"The novelty of this work is that we are trying to convert CO2 into something that is value-added but in a solid, useful form," Chen said.
Such solid carbon materials—including carbon nanotubes and nanofibers with dimensions measuring billionths of a meter—have many appealing properties, including strength and thermal and electrical conductivity. But it's no simple matter to extract carbon from carbon dioxide and get it to assemble into these fine-scale structures. One direct, heat-driven process requires temperatures in excess of 1,000 degrees Celsius.
"It's very unrealistic for large-scale CO2 mitigation," Chen said. "In contrast, we found a process that can occur at about 400 degrees Celsius, which is a much more practical, industrially achievable temperature."
The tandem two-step
The trick was to break the reaction into stages and to use two different types of catalysts—materials that make it easier for molecules to come together and react.
"If you decouple the reaction into several sub-reaction steps you can consider using different kinds of energy input and catalysts to make each part of the reaction work," said Brookhaven Lab and Columbia research scientist Zhenhua Xie, lead author on the paper.
The scientists started by realizing that carbon monoxide (CO) is a much better starting material than CO2 for making carbon nanofibers (CNF). Then they backtracked to find the most efficient way to generate CO from CO2.
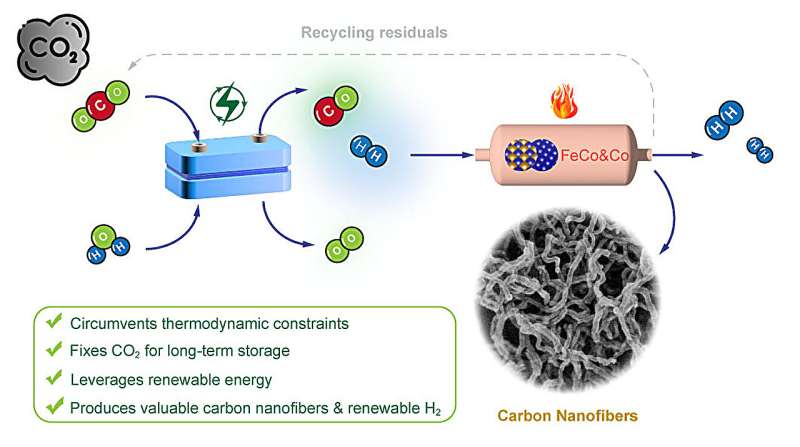
Earlier work from their group steered them to use a commercially available electrocatalyst made of palladium supported on carbon. Electrocatalysts drive chemical reactions using an electric current. In the presence of flowing electrons and protons, the catalyst splits both CO2 and water (H2O) into CO and H2.
For the second step, the scientists turned to a heat-activated thermocatalyst made of an iron-cobalt alloy. It operates at temperatures around 400 degrees Celsius, significantly milder than a direct CO2-to-CNF conversion would require. They also discovered that adding a bit of extra metallic cobalt greatly enhances the formation of the carbon nanofibers.
"By coupling electrocatalysis and thermocatalysis, we are using this tandem process to achieve things that cannot be achieved by either process alone," Chen said.
Catalyst characterization
To discover the details of how these catalysts operate, the scientists conducted a wide range of experiments. These included computational modeling studies, physical and chemical characterization studies at Brookhaven Lab's National Synchrotron Light Source II (NSLS-II)—using the Quick X-ray Absorption and Scattering (QAS) and Inner-Shell Spectroscopy (ISS) beamlines—and microscopic imaging at the Electron Microscopy facility at the Lab's Center for Functional Nanomaterials (CFN).
On the modeling front, the scientists used "density functional theory" (DFT) calculations to analyze the atomic arrangements and other characteristics of the catalysts when interacting with the active chemical environment.
"We are looking at the structures to determine what are the stable phases of the catalyst under reaction conditions," explained study co-author Ping Liu of Brookhaven's Chemistry Division who led these calculations. "We are looking at active sites and how these sites are bonding with the reaction intermediates. By determining the barriers, or transition states, from one step to another, we learn exactly how the catalyst is functioning during the reaction."
X-ray diffraction and X-ray absorption experiments at NSLS-II tracked how the catalysts changed physically and chemically during the reactions. For example, synchrotron X-rays revealed how the presence of electric current transforms metallic palladium in the catalyst into palladium hydride, a metal that is key to producing both H2 and CO in the first reaction stage.
For the second stage, "We wanted to know what's the structure of the iron-cobalt system under reaction conditions and how to optimize the iron-cobalt catalyst," Xie said. The X-ray experiments confirmed that both an alloy of iron and cobalt plus some extra metallic cobalt are present and needed to convert CO to carbon nanofibers.
"The two work together sequentially," said Liu, whose DFT calculations helped explain the process.
"According to our study, the cobalt-iron sites in the alloy help to break the C-O bonds of carbon monoxide. That makes atomic carbon available to serve as the source for building carbon nanofibers. Then the extra cobalt is there to facilitate the formation of the C-C bonds that link up the carbon atoms," she explained.
Recycle-ready, carbon-negative
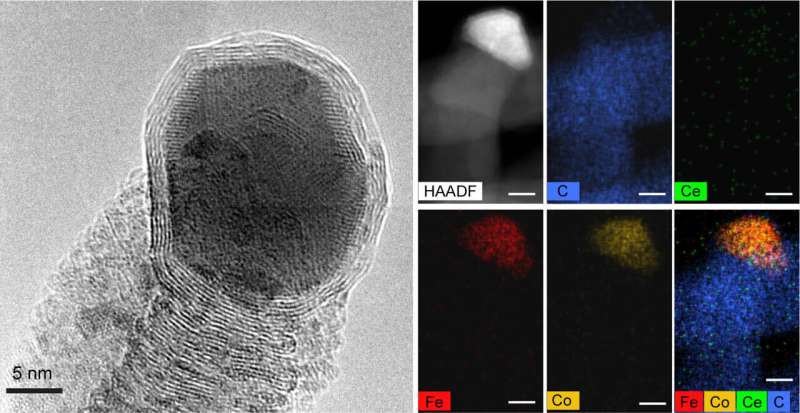
"Transmission electron microscopy (TEM) analysis conducted at CFN revealed the morphologies, crystal structures, and elemental distributions within the carbon nanofibers both with and without catalysts," said CFN scientist and study co-author Sooyeon Hwang.
The images show that, as the carbon nanofibers grow, the catalyst gets pushed up and away from the surface. That makes it easy to recycle the catalytic metal, Chen said.
"We use acid to leach the metal out without destroying the carbon nanofiber so we can concentrate the metals and recycle them to be used as a catalyst again," he said.
This ease of catalyst recycling, commercial availability of the catalysts, and relatively mild reaction conditions for the second reaction all contribute to a favorable assessment of the energy and other costs associated with the process, the researchers said.
"For practical applications, both are really important—the CO2 footprint analysis and the recyclability of the catalyst," said Chen. "Our technical results and these other analyses show that this tandem strategy opens a door for decarbonizing CO2 into valuable solid carbon products while producing renewable H2."
If these processes are driven by renewable energy, the results would be truly carbon-negative, opening new opportunities for CO2 mitigation.
More information: CO2 fixation into carbon nanofibres using electrochemical–thermochemical tandem catalysis, Nature Catalysis (2024). www.nature.com/articles/s41929-023-01085-1
Citation: Catalytic combo converts CO₂ to solid carbon nanofibers while offsetting emissions (2024, January 11) retrieved 11 January 2024 from https://techxplore.com/news/2024-01-catalytic-combo-solid-carbon-nanofibers.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
