A new technology can extract lithium from brines at an estimated cost of under 40% that of today's dominant extraction method, and at just a fourth of lithium's current market price. The new technology would also be much more reliable and sustainable in its use of water, chemicals, and land than today's technology, according to a study published in Matter by Stanford University researchers.
Global demand for lithium has surged in recent years, driven by the rise of electric vehicles and renewable energy storage. The dominant source of lithium extraction today relies on evaporating brines in huge ponds under the sun for a year or more, leaving behind a lithium-rich solution, after which heavy use of potentially toxic chemicals finishes the job. Water with a high concentration of salts, including lithium, occurs naturally in some lakes, hot springs, and aquifers, and as a byproduct of oil and natural gas operations and of seawater desalination.
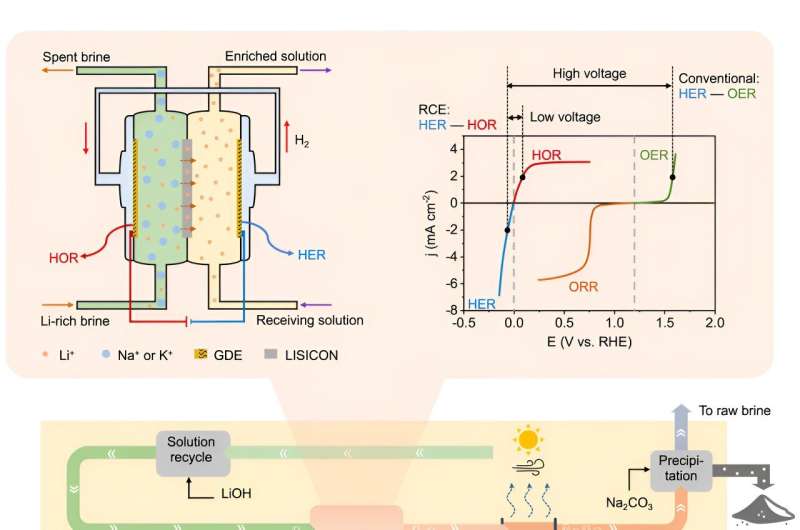
Many scientists are searching for less expensive and more efficient, reliable, and environmentally friendly lithium extraction methods. These are generally direct lithium extraction that bypasses big evaporation ponds. The new study reports on the results of a new method using an approach known as "redox-couple electrodialysis," or RCE, along with cost estimates.
"The benefits to efficiency and cost innate to our approach make it a promising alternative to current extraction techniques and a potential game changer for the lithium supply chain," said Yi Cui, the study's senior author and a professor of materials science and engineering in the School of Engineering.
The research team estimates that its approach costs $3,500 to $4,400 per ton of high-purity lithium hydroxide, which can be converted to battery-grade lithium carbonate inexpensively, compared with costs of about $9,100 per ton for the dominant technology for extracting lithium from brine. The current market price for battery-grade lithium carbonate is almost $15,000 per ton, but a shortage in late 2022 drove the volatile lithium market price to $80,000.
Meeting growing demand
Lithium, so far, has had a critical role in the global transition to sustainable energy. The demand for lithium is expected to rise from approximately half a million metric tons in 2021 to an estimated 3 million to 4 million metric tons by 2030, according to a report by McKinsey & Co. This sharp increase is driven mostly by the rapid adoption of electric vehicles and renewable energy storage systems, both of which rely heavily on batteries.
Traditionally, lithium has been extracted from mined rocks, a method that is even more expensive, energy intensive, and driven by toxic chemicals than brine extraction. As a result, the dominant method for lithium extraction today has switched to evaporating salt-lake brines, though still at high financial and environmental costs. This method is also heavily dependent on specific climatic conditions that limit the number of commercially viable salt lakes, throwing into doubt the lithium industry's ability to meet rising demand.
The new method from Cui and his team uses electricity to move lithium through a solid-state electrolyte membrane from water with a low lithium concentration to a more concentrated, high-purity solution. Each of a series of cells increases the lithium concentration to a solution from which final chemical isolation is relatively easy. This approach uses less than 10% of the electricity required by current brine extraction technology and has a lithium selectivity of almost 100%, making it very efficient.
"The advantages displayed by our approach over conventional lithium extraction techniques enhance its feasibility in eco-friendly and cost-effective lithium production," said co-lead author of the study, Rong Xu, a former postdoctoral researcher in Cui's lab, now a faculty member at Xi'an Jiaotong University in China. "Eventually, we hope our method will significantly advance electrified transportation and the ability to store renewable energy."
Cost and environmental benefits
The study includes a brief techno-economic analysis comparing the costs of current lithium extraction with those of the RCE approach. The new method is expected to be relatively inexpensive due mostly to lower capital costs. It eliminates the need for large-scale solar evaporation ponds, which are expensive to build and maintain. The new method's use of significantly less electricity, water, and chemical agents—aside from the sustainability benefits—further lowers costs.
By avoiding the extensive land use and water consumption of traditional methods, the RCE approach also reduces the ecological footprint of lithium production.
The RCE method works with a variety of saline waters, including those with varying concentrations of lithium, sodium, and potassium. Study experiments showed that the new technology could extract lithium, for example, from wastewater resulting from oil production. It could potentially be used to extract lithium from seawater, which has lower lithium concentrations than brines. Lithium extraction from seawater using conventional methods is not commercially viable today.
"Direct lithium extraction techniques like ours have been in development for a while. The main contending technologies to date have significant drawbacks, like the inability to operate continuously, high energetic costs, or relatively low efficiency," said Ge Zhang, a Stanford postdoctoral scholar and co-author of the study. "Our method seems to have none of these drawbacks. Its continuous operation could contribute to a more reliable lithium supply and calm the volatile lithium market."
Looking ahead
The scalability of the RCE method is also promising. In experiments where the scale of the device was increased fourfold, the RCE method continued to perform well, with both energy efficiency and lithium selectivity remaining very high.
"This suggests that the method could be applied on an industrial scale, making it a viable alternative to current extraction technologies," said Cui.
Nevertheless, the study highlights some areas for further research and development. The researchers experimented with two versions of their method. One extracted the lithium more quickly and used more electricity. The other was slower and used less electricity. The slower extraction resulted in lower costs and a more stable membrane for extracting the lithium continuously and for a long time, compared with the faster extraction. Under high current densities and faster water flow, the membranes degraded, leading to reduced efficiency over time.
Even though this was not evident in the slower extracting experiment, the researchers want to optimize the design of their device for potentially faster extraction. They are already testing other promising materials for the membrane.
Also, the researchers did not demonstrate lithium extraction from seawater in this study.
"In principle, our method is applicable for seawater as well, but there could be stability problems for the membrane in seawater," said Zhang.
Still, the team remains quite optimistic.
"As our research continues, we think our method could soon move from the laboratory to large-scale industrial applications," said Xu.
More information: Rong Xu et al, Continuous Lithium Extraction from Brine by Efficient Redox-Couple Electrodialysis, Matter (2024). DOI: 10.1016/j.matt.2024.07.014. www.sciencedirect.com/science/ … ii/S2590238524004247
Citation: New technology extracts lithium from brines inexpensively and sustainably (2024, August 21) retrieved 21 August 2024 from https://techxplore.com/news/2024-08-technology-lithium-brines-inexpensively-sustainably.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
