The struggle to cut emissions is real. Last year, the world emitted more than 37 billion metric tons of carbon dioxide, setting a new record high. As a result, sucking CO2 out of the atmosphere has become an increasingly popular idea. Governments worldwide are banking on this technology, called direct air capture, to help them achieve climate goals and avoid the worst consequences of climate change.
But despite more than a dozen direct air capture facilities being up and running around the globe already, the technology still faces major technological hurdles—including its own high energy use.
In a study published May 1 in the journal ACS Energy Letters, researchers at the University of Colorado Boulder and collaborators revealed that a popular approach many engineers are exploring to reduce those energy costs would, in reality, fail. The team, including scientists at the National Renewable Energy Laboratory in Golden, Colorado and Delft University of Technology in the Netherlands, also proposed an alternative, more sustainable design for capturing CO2 and converting it to fuels.
"Ideally, we want to take CO2 out of the air and keep it out of the air," said first author Hussain Almajed, a Ph.D. student in the Department of Chemical and Biological Engineering. "However, some of this CO2 can be recycled into useful carbon-containing products, which is why researchers have proposed different ideas of how we can achieve that. Some of these ideas look very simple and elegant on paper, but researchers rarely check whether they are practical and economical in industrial settings."
Trapping the gas
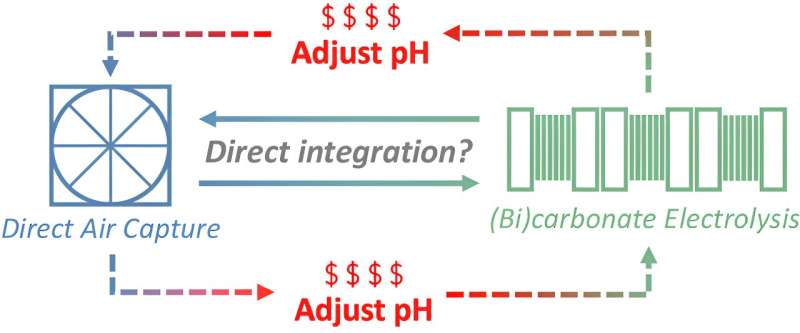
One of the most common direct air capture approaches is to use air contactors, essentially huge fans that pull air into a chamber filled with a basic liquid. CO2 is acidic, so it naturally binds to and reacts with the solution to form harmless carbonate (the main ingredient in concrete) or bicarbonate (the ingredient in baking soda).
Stratos, one of the world's largest direct air capture facilities under construction in Texas, uses this approach.
Once CO2 is trapped in the carbonate or bicarbonate solutions, engineers must separate it out from the liquid so the liquid can return to the chamber to capture more CO2.
Meanwhile, the captured carbon can be converted into things like plastics, carbonated drinks and even—with further processing—fuel to power homes and potentially airplanes.
But there is a catch. To release the trapped CO2, companies need to heat the carbonate and bicarbonate solution to at least 900˚C (1,652° F), a temperature solar and wind energy is unable to achieve. This step is usually powered by burning fossil-based fuels like natural gas or pure methane.
"If we have to release CO2 in order to capture CO2, it defeats the whole purpose of carbon capture," said Wilson Smith, a professor in the Department of Chemical and Biological Engineering and a fellow of the Renewable and Sustainable Energy Institute at CU Boulder.
Close the loop
Researchers are actively looking for answers. One idea, commonly known as reactive capture, is to apply electricity to the carbonate and bicarbonate solutions, zapping the CO2 and basic liquid apart in the chamber. In theory, the recycled liquid can then capture more CO2, forming a closed-loop system.
"Reactive capture is now the buzzword in the field, and researchers proposed that it could help save energy and costs associated with carbon capture. But no one really assessed whether that's realistic under industrial conditions," Almajed said.
To do that, the team calculated the mass and energy outputs of the reactive capture units, based on given inputs, to understand how well the overall system would perform. They found that in an industrial setting, electricity would not be able to regenerate the basic liquid to re-capture more CO2 from the air.
In fact, after five cycles of carbon capture and regeneration, the basic liquid could barely pull any CO2 out of the air.
The team also suggested a tweak to the reactive capture process by adding a step called electrodialysis. The process splits additional water into acidic and basic ions, helping to maintain the basic liquid's ability to absorb more CO2. Electrodialysis can run on renewable electricity, making it a potentially sustainable way to turn captured CO2 into useful products.
More importantly, electrodialysis can release CO2 gas, which engineers can use to strengthen concrete.
"To me, turning CO2 into rocks has to be one of the leading solutions to keep it out of the air over long periods of time," Smith said. Concrete production is energy-intensive and responsible for 8% of global carbon emissions.
"This is solving multiple problems with one technology," he said.
The root of the problem
According to the Intergovernmental Panel on Climate Change (IPCC), a team of scientists convened by the United Nations, carbon dioxide removal "is required to achieve global and national targets of net zero CO2 and greenhouse gas emissions."
Across the world, more than 20 direct air capture plants are in operation with 130 more currently under construction.
But Smith stresses that while carbon capture may have its place, cutting emissions is still the most critical step needed to avoid the worst outcomes of climate change.
"Imagining Earth as a bathtub, with the running water from the faucet being CO2. The bathtub is getting full and becoming unlivable. Now, we have two options. We can use a little cup to scoop out the water, cup by cup, or we can turn the faucet off," Smith said.
"Cutting emissions has to be the priority."
More information: Hussain M. Almajed et al, Closing the Loop: Unexamined Performance Trade-Offs of Integrating Direct Air Capture with (Bi)carbonate Electrolysis, ACS Energy Letters (2024). DOI: 10.1021/acsenergylett.4c00807
Citation: Converting captured carbon to fuel: Study assesses what's practical and what's not (2024, July 22) retrieved 22 July 2024 from https://techxplore.com/news/2024-07-captured-carbon-fuel.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
